What happens when you add heat energy to water? To see how heat changes the motion of molecules, follow this simple experiment by adding food coloring to hot water and cold water.
What happens when you add pressure to ice? {When pressure is added to ice, its melting point is reduced.} Check out this experiment (video) to see how it works. We used two knives attached to strings to apply the pressure to our ice. (In hindsight, we should have done this in a colder climate because the entire cube of ice was melting inside our very warm home. In the above photo, you can only barely see where the ice was melting more quickly where pressure was applied. The video is much better at showing this. In hindsight, I also should have cleaned the counters before taking the photos.)
What happens when you put salt onto a piece of ice? When salt is added to ice, it reduces its melting point. (The ice melts at a lower temperature, which is why they salt the roads before ice/snow storms.)
Plasma, by definition, is (in physics)
One of four main states of matter, similar to a gas, but consisting of positively charged ions with most or all of their detached electrons moving freely about. Plasmas are produced by very high temperatures, as in the Sun and other stars, and also by the ionization resulting from exposure to an electric current, as in a fluorescent light bulb or a neon sign. (Science Dictionary definition at dictionary.com)
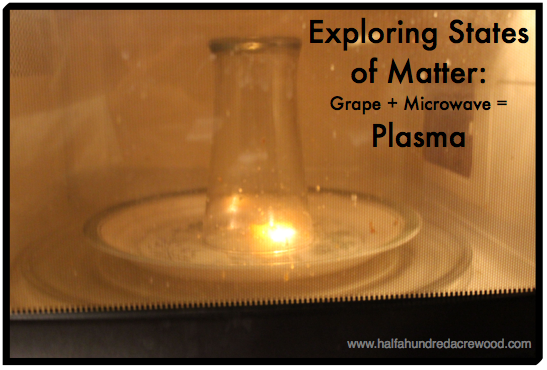
So… how can we experiment with plasma? First, we need something that can provide us with ions, and second, we need very high temperatures. You can heat up a grape in the microwave. PLEASE USE CAUTION if you try this experiment!
Learn more about States of Matter at NeoK12.